Chapter 7: Lipids
7.3 Triglycerides
University of Hawai‘i at Mānoa Food Science and Human Nutrition Program
Lipids are unique organic compounds, each serving key roles and performing specific functions within the body. There are three main types of lipids in our bodies and in our food:
- Triglycerides
- Phospholipids
- Sterols
Triglycerides are the most common lipid in our bodies and in the foods we consume. We’ll talk about triglycerides first and come back to phospholipids and sterols in a later section.
Triglycerides Structure and Functions
Triglycerides are the main form of lipid found in the body and in the diet. Fatty acids and glycerol are the building blocks of triglycerides. Glycerol is a thick, smooth, syrupy compound that is often used in the food industry. To form a triglyceride, a glycerol molecule is joined by three fatty acid chains. triglycerides contain varying mixtures of fatty acids. Breaking down the name triglyceride tells a lot about their structure. “Tri” refers to the three fatty acids, “glyceride” refers to the glycerol backbone that the three fatty acids are bonded to. You can also have monoglycerides with one fatty acid and diglycerides that contain two fatty acids.
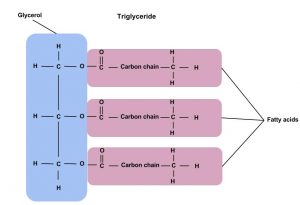
Fatty Acids
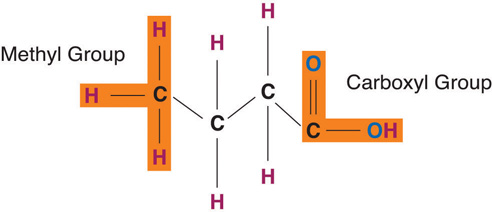
Fatty acids determine if the compound is solid or liquid at room temperature. Fatty acids consist of a carboxylic acid (−COOH) group on one end of a carbon chain and a methyl group (−CH3) on the other end. Fatty acids can differ from one another in two important ways—carbon chain length and degree of saturation. The three fatty acids in a triglyceride can be the same or can each be a different fatty acid.
It’s All in the Chain
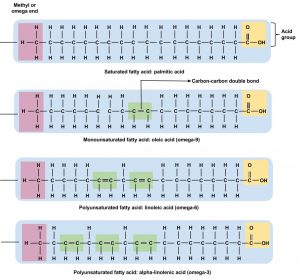
Fatty acids have different chain lengths and different compositions. Foods have fatty acids with chain lengths between four and twenty-four carbons and most of them contain an even number of carbon atoms. When the carbon chain length is shorter, the melting point of the fatty acid becomes lower—and the fatty acid becomes more liquid.
Fatty Acid Types in the Body
The fatty-acid profile of the diet directly correlates to the tissue lipid profile of the body. It may not solely be the quantity of dietary fat that matters. More directly, the type of dietary fat ingested has been shown to affect body weight, composition, and metabolism. The fatty acids consumed are often incorporated into the triglycerides within the body. Evidence confirms that saturated fatty acids are linked to higher rates of weight retention when compared to other types of fatty acids. Alternatively, the fatty acids found in fish oil are proven to reduce the rate of weight gain as compared to other fatty acids.[1]
Degrees of Saturation
Fatty acid chains are held together by carbon atoms that attach to each other and to hydrogen atoms. The term saturation refers to whether or not a fatty acid chain is filled (or “saturated”) to capacity with hydrogen atoms. If each available carbon bond holds a hydrogen atom we call this a saturated fatty acid chain. All carbon atoms in such a fatty acid chain are bonded with single bonds. Sometimes the chain has a place where hydrogen atoms are missing. This is referred to as the point of unsaturation.
When one or more bonds between carbon atoms are a double bond (C=C), that fatty acid is called an unsaturated fatty acid, as it has one or more points of unsaturation. Any fatty acid that has only one double bond is a monounsaturated fatty acid, an example of which is olive oil (75 percent of its fat is monounsaturated). Monounsaturated fats help regulate blood cholesterol levels, thereby reducing the risk for heart disease and stroke. A polyunsaturated fatty acid is a fatty acid with two or more double bonds or two or more points of unsaturation. Soybean oil contains high amounts of polyunsaturated fatty acids. Both monounsaturated fats and polyunsaturated fats provide nutrition that is essential for normal cell development and healthy skin.
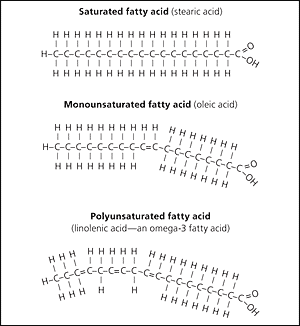
Foods that have a high percentage of saturated fatty acids tend to be solid at room temperature. Examples of these are fats found in chocolate (stearic acid, an eighteen-carbon saturated fatty acid is a primary component) and meat. Foods rich in unsaturated fatty acids, such as olive oil (oleic acid, an eighteen-carbon unsaturated fatty acid, is a major component) tend to be liquid at room temperature. Flaxseed oil is rich in alpha-linolenic acid, which is an unsaturated fatty acid and becomes a thin liquid at room temperature.
Knowing the connection between chain length, degree of saturation, and the state of the fatty acid (solid or liquid) is important for making food choices. If you decide to limit or redirect your intake of fat products, then choosing unsaturated fat is more beneficial than choosing a saturated fat. This choice is easy enough to make because unsaturated fats tend to be liquid at room temperature (for example, olive oil) whereas saturated fats tend to be solid at room temperature (for example, butter). Avocados are rich in unsaturated fats. Most vegetable and fish oils contain high quantities of polyunsaturated fats. Olive oil and canola oil are also rich in monounsaturated fats. Conversely, tropical oils are an exception to this rule in that they are liquid at room temperature yet high in saturated fat. Palm oil (often used in food processing) is highly saturated and has been proven to raise blood cholesterol. Shortening, margarine, and commercially prepared products (in general) report to use only vegetable-derived fats in their processing. But even so, much of the fat they use may be in the saturated and trans fat categories.
Cis or Trans Fatty Acids?
The introduction of a carbon double bond in a carbon chain, as in an unsaturated fatty acid, can result in different structures for the same fatty acid composition. When the hydrogen atoms are bonded to the same side of the carbon chain, it is called a cis fatty acid. Because the hydrogen atoms are on the same side, the carbon chain has a bent structure. Naturally occurring fatty acids usually have a cis configuration.
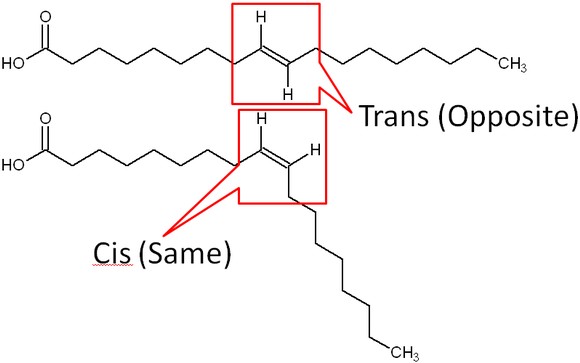
In a trans fatty acid, the hydrogen atoms are attached on opposite sides of the carbon chain. There are some naturally occurring trans fatty acids, such as conjugated linoleic acid (CLA), in dairy products. However, for the most part, trans fatty acids in our diets are not natural; instead, they have been produced synthetically. The primary source of trans fatty acids in our food supply is partially hydrogenated vegetable oil. The ‘hydrogenated’ means that the oil has gone through the process of hydrogenation. Hydrogenation, like the name implies, is the addition of hydrogen. This is how vegetable oils are converted into semisolid fats for use in the manufacturing process.
If an unsaturated fatty acid is completely hydrogenated it would be converted to a saturated fatty acid as shown in Figure 7.35.
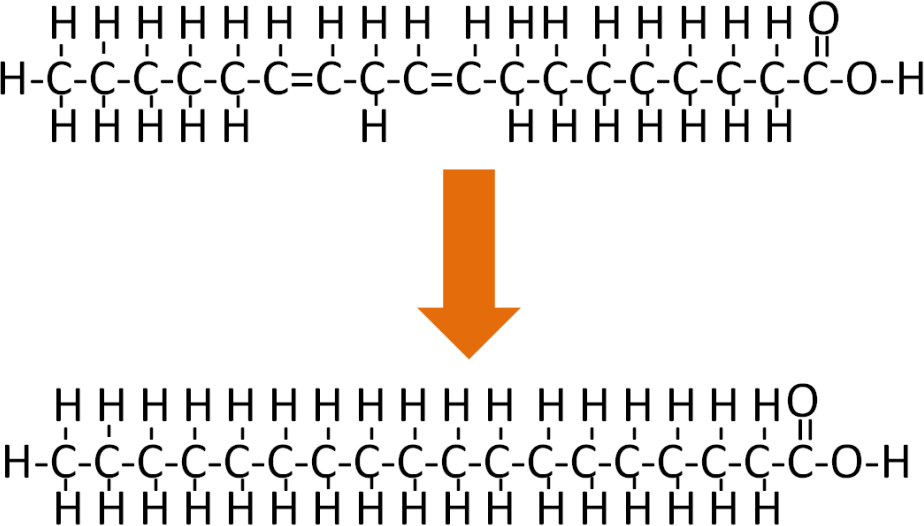
However, complete hydrogenation isn’t/wasn’t always desirable, thus partially hydrogenated vegetable oil became widely used. To visualize the difference in the amount of hydrogenation consider the difference between tub margarine and stick margarine.
Stick margarine is more fully hydrogenated giving it a more solid texture. This is one of the two reasons to hydrogenate, to get a more solid texture. The second reason is that it makes it more shelf-stable, because the double bond(s) of unsaturated fatty acids are susceptible to oxidation, which causes them to become rancid.
Partial hydrogenation causes the conversion of cis to trans fatty acids along with the formation of some saturated fatty acids. Originally, it was thought that trans fatty acids would be a better alternative to saturated fat (think margarine vs. butter). However, it turns out that trans fat is actually worse than saturated fat in altering biomarkers associated with cardiovascular disease. Trans fat increases LDL and decreases HDL levels, while saturated fat increased LDL without altering HDL levels. But this does not mean that butter is a better choice than margarine as described in the first link. The FDA revoked Generally Recognized as Safe (GRAS) status of partially hydrogenated vegetable oil in 2015, requiring its use to be phased out by 2018.[2]
Interestingly, some naturally occurring trans fats do not pose the same health risks as their artificially engineered counterparts. These trans fats are found in ruminant animals such as cows, sheep, and goats, resulting in trans fatty acids being present in our meat, milk, and other dairy product supply. Reports from the US Department of Agriculture (USDA) indicate that these trans fats comprise 15 to 20 percent of the total trans-fat intake in our diet. While we know that trans fats are not exactly harmless, it seems that any negative effect naturally occurring trans fats have are counteracted by the presence of other fatty acid molecules in these animal products, which work to promote human health.
- Mori T, Kondo H. Dietary fish oil upregulates intestinal lipid metabolism and reduces body weight gain in C57BL/6J mice. J Nutr. 2007;137(12):2629-34. DOI: 10.1093/jn/137.12.2629 ↵
- https://www.hsph.harvard.edu/news/hsph-in-the-news/us-bans-artificial-trans-fats/ ↵
“heart disease” refers to several types of heart conditions. The most common type is coronary artery disease, which can cause a heart attack. Other kinds of heart disease may involve the valves in the heart, or the heart may not pump well and cause heart failure.
Food processing involves transforming raw ingredients into packaged food, from fresh-baked goods to frozen dinners.
The Federal Food, Drug, and Cosmetic Act of 1938 gives the FDA authority over food ingredients. The FDA enforces the safety of domestic and imported foods. It also monitors supplements, food labels, claims that corporations make about the benefits of products, and pharmaceutical drugs. Sometimes, the FDA must recall contaminated foods and remove them from the market to protect public health.
The USDA develops and executes federal policy on farming and food. This agency supports farmers and ranchers, protects natural resources, promotes trade, and seeks to end hunger in the United States and abroad. The USDA also oversees the regulation of meat, poultry, and processed egg products.

