Chapter 10: Micronutrients Overview and Role as Antioxidants
10.3 Antioxidants
The antioxidant vitamins and minerals include the following:
- Vitamin E
- Vitamin C
- Selenium
- Iron
- Copper
- Zinc
- Manganese
- Riboflavin
In this section, we are going to cover vitamin E, vitamin C, and selenium in detail because being an antioxidant is their primary function. Iron, copper, and zinc will be discussed in chapter 12, with other blood micronutrients. Manganese and riboflavin will be discussed in chapter 11, with other micronutrients involved in metabolism.
Free Radicals & Oxidative Stress
Before you can understand what an antioxidant is, it is important to have an understanding of oxidants. As you learned in chapter 8, oxidation is the loss of an electron. (See Figure 10.31 below for a reminder.) An oxidant is a molecule that causes oxidation of other molecules. When this happens, the other molecule is said to become oxidized. In other words, an oxidant is a molecule that takes electrons away from other molecules. Oxidation is a normal part of many important metabolic pathways in the cell, including those pathways that produce ATP. However, if too much oxidation occurs, essential molecules can become irreparably damaged.
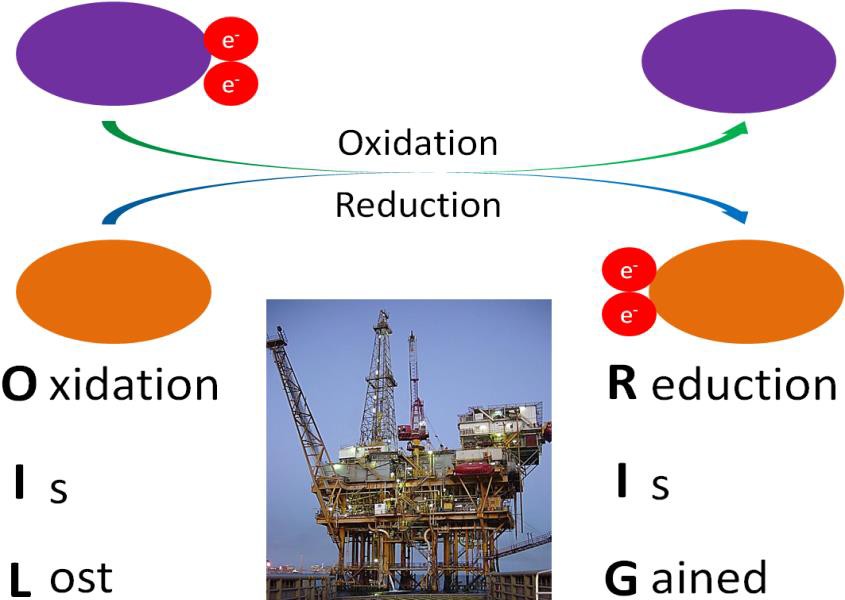
Some important terms to understand:
Free Radical – a molecule with an unpaired electron in its outer orbital.
The following example shows normal oxygen losing an electron from its outer orbital and thus, becoming an oxygen free radical.
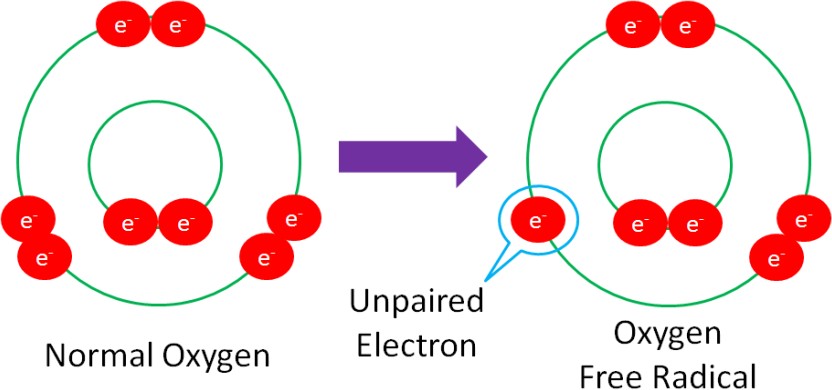
Free radicals are highly reactive because they actively seek an electron to stabilize (pair with) the unpaired electron within the molecule. This makes free radicals very strong oxidants.
Reactive Oxygen Species (ROS) – an oxygen-containing free radical species. Some of the most common ROS are (● symbolizes radical):
- Superoxide (O ●)
- Hydroxyl Radical (●OH)
- Hydrogen Peroxide Radical (HO ●)
- Peroxyl Radical (ROO ●)
- Alkoxyl Radical (RO●)
- Ozone (O3)
- Singlet Oxygen (1O2)
- Hydrogen Peroxide (H2O2)
Oxidative Stress – the imbalance between the production of ROS/free radicals and the body’s ability to quench them. In other words, oxidative stress is what your cells experience when you’re making more free radicals than your cells can handle.
Free radicals can be generated by a variety of sources both within the body and outside the body. The figure below shows some of the sources of free radicals.
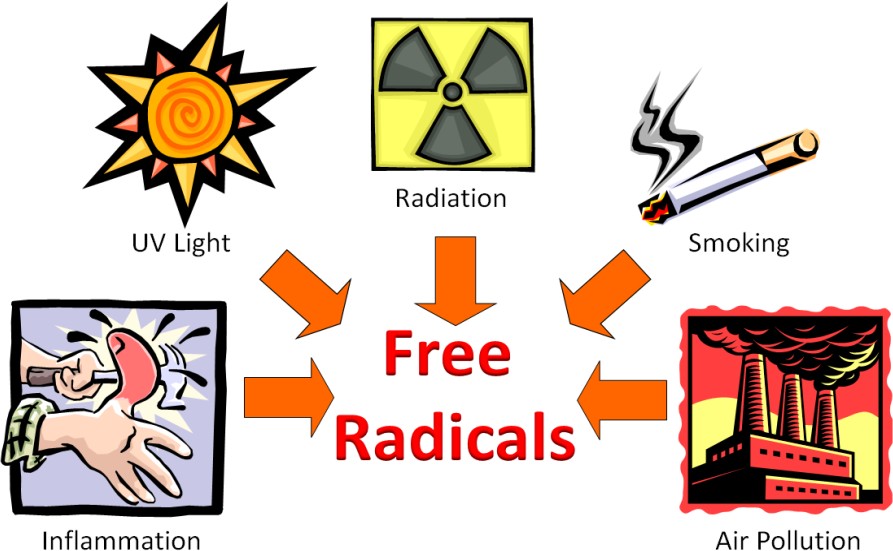
The Web Link below does a good job explaining what oxidative stress is, how free radicals can be formed, how they are neutralized by antioxidants, where we get antioxidants.
Video Link: Oxidative Stress, Free Radicals, & Antioxidants
So, we have these free radicals searching for an electron, what’s the big deal? The problem arises if the free radicals oxidize low-density lipoproteins (LDLs), proteins, or DNA.
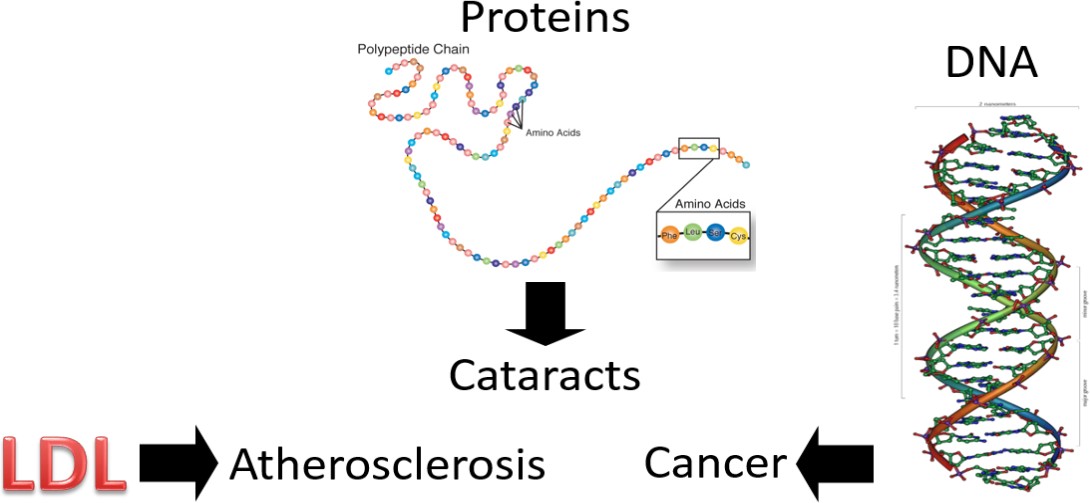
Oxidized LDL is more likely to contribute to atherosclerosis (hardening of the arteries) than normal LDL. Protein oxidation is believed to be involved in the development of cataracts. Cataracts are the clouding of the lens of the eye, which can lead to blindness if not corrected. If DNA becomes oxidized, it can result in a mutation. A mutation is a change in the nucleotide or base pair sequence of DNA. If enough mutations occur, or if they occur in the wrong place, they can lead to cancer. You may have noticed that at least a few of the sources of free radicals in figure 10.33 are known to cause cancer.
What is an Antioxidant?
We are now ready to move on to antioxidants, which as their name indicates, combat free radicals, ROS, and oxidative stress. As a humorous introduction, the link below is to a cartoon that shows Auntie Oxidant kicking free radicals out of the bloodstream.
Web Link: Auntie Oxidant
Unfortunately, it’s not quite that simple. You have probably heard the saying “take one for the team.” Instead of taking one for the team, antioxidants “give one for the team.” The ‘giving’ in this example is the donation of an electron from itself to a free radical, in order to regenerate a stable compound, as shown in Figure 10.35.
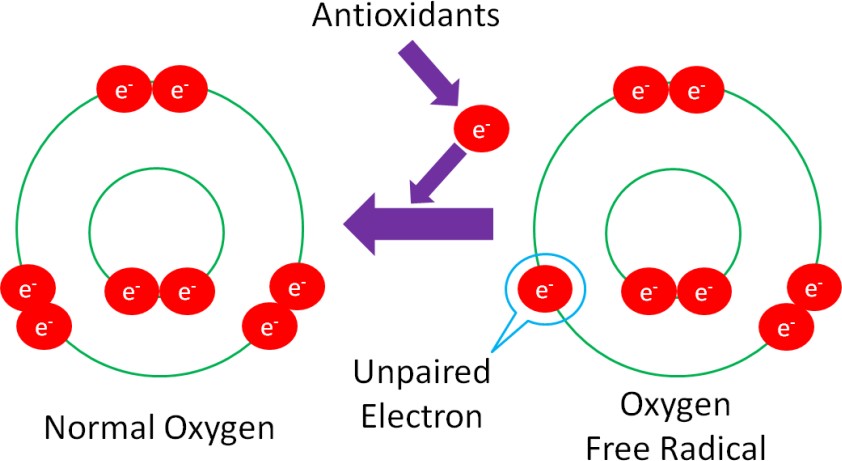
Donating an electron is how vitamins (A, C & E) act as antioxidants. Minerals, on the other hand, are not antioxidants themselves. Instead, they are cofactors for antioxidant enzymes.
These antioxidant enzymes include:
- Superoxide dismutase (SOD): uses copper, zinc, and manganese as cofactors (there is more than one SOD enzyme); converts superoxide to hydrogen peroxide and oxygen.[1]
- Catalase: uses iron as a cofactor; converts hydrogen peroxide to water.[2]
- Glutathione peroxidase (GPX): is a selenoenzyme that converts hydrogen peroxide to water. It can also convert other reactive oxygen species (ROSs) to water.[3]
- Peroxiredoxin: participates directly in eliminating hydrogen peroxide (H2O2) and neutralizing other reactive oxygen species (ROS).[4]
Antioxidants are thought to work in concert with one another, forming what is known as the antioxidant network. For example, vitamin E, vitamin C, and selenium often work together to process a single reactive oxygen species.
Meaningful Antioxidant(s)
There is a lot of confusion among the public on antioxidants. For the most part, this is for a good reason. Many food companies put antioxidant numbers on the packages that sound good to consumers, who often have no idea how to interpret them. Thus, it is increasingly important to have an understanding of what a meaningful antioxidant actually is.
A meaningful antioxidant has two characteristics (these are based on the assumption that the compound is an antioxidant):
- Found in appreciable amounts in a location where there are free radicals/ROS that need to be quenched
- It is not redundant with another antioxidant that is already providing that function
What do these mean? Let’s consider the example of lycopene and vitamin E, which are both fat-soluble antioxidants. In a lab setting (in vitro), lycopene has been shown to be 10x more effective than vitamin E. However, when you look at the concentrations found in the body, there is far more vitamin E (alpha-tocopherol) than lycopene:[5]
- LDL – 13x more alpha-tocopherol than lycopene.
- Prostate – 162x higher alpha-tocopherol than lycopene concentrations
- Skin – 17 to 269x higher alpha-tocopherol than lycopene concentrations
- Plasma – 53x higher alpha-tocopherol than lycopene concentrations
Thus, despite the fact that lycopene is a better antioxidant in the laboratory, vitamin E is likely the more meaningful antioxidant in the body, as evidenced by the fact that its concentration so much higher in the various tissues (locations of need.) In addition, if lycopene and alpha-tocopherol had similar antioxidant functions, lycopene’s potential antioxidant action is redundant to alpha-tocopherol’s antioxidant function and thus, lycopene is less likely to be a meaningful antioxidant.
Too Much of a Good Thing? Antioxidants as Pro-oxidants
A clinical trial once found that high-dose beta-carotene supplementation increased lung cancer risk in smokers.[6] This is an example of findings that support that high doses of antioxidants may be “too much of a good thing”, causing more harm than benefit. The parabolic, or U-shaped figure, below displays how the level of nutrient concentration or intake (horizontal axis) relates to an antioxidant measure (vertical axis). The lowest level of antioxidant intake or tissue concentration results in nutrient deficiency if the antioxidant is essential (vitamins and minerals). Intake levels above deficient, but less than optimal, are referred to as low suboptimal. Suboptimal means the levels are not optimal. Thus, low suboptimal and high suboptimal sandwich optimal. The high suboptimal level is between optimal and where the nutrient becomes toxic.
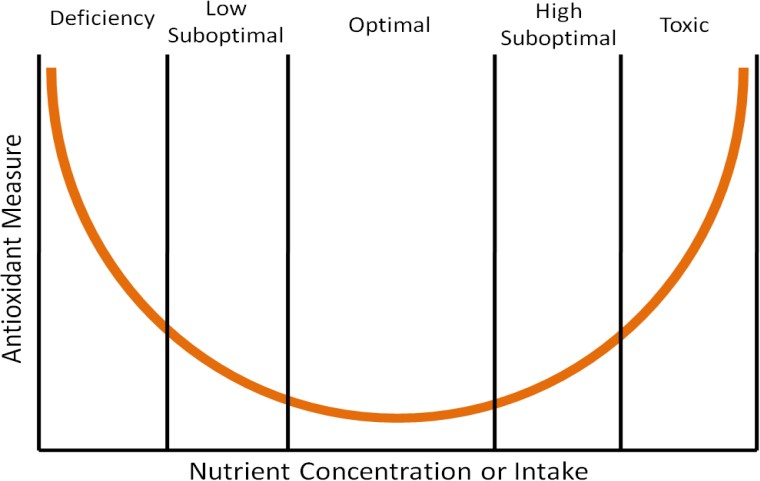
Another example of this phenomenon can be seen when we look at DNA damage in the prostate gland of dogs as it relates to toenail selenium concentration measurements, which are a good indicator of long-term selenium status.[7] Researchers found that when they plotted prostate DNA damage (a measure of oxidative stress) against toenail selenium status (nutrient concentration or intake) that it resulted in a U-shaped curve like the one shown above. Thus, it is good to have antioxidants in your diet, but too much can be counterproductive.
- Gropper SS, Smith JL, Groff JL. (2008) Advanced Nutrition and Human Metabolism. Belmont, CA: Wadsworth Publishing. ↵
- Gropper SS, Smith JL, Groff JL. (2008) Advanced Nutrition and Human Metabolism. Belmont, CA: Wadsworth Publishing. ↵
- Gropper SS, Smith JL, Groff JL. (2008) Advanced Nutrition and Human Metabolism. Belmont, CA: Wadsworth Publishing. ↵
- Yuan J, Murrell GA, Trickett A, Landtmeters M, Knoops B, Wang MX. (2004) Overexpression of antioxidant enzyme peroxiredoxin 5 protects human tendon cells against apoptosis and loss of cellular function during oxidative stress. Biochim Biophys Acta. 1693(1):37-45. DOI: 10.1016/j.bbamcr.2004.04.006. ↵
- Erdman, J.W., Ford, N.A., Lindshield, B.L. Are the health attributes of lycopene related to its antioxidant function? Arch Biochem Biophys, 483: 229-235, 2009. ↵
- Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature 290, 201-208, 1981. ↵
- Waters DJ, Shen S, Glickman LT, Cooley DM, Bostwick DG, et al. (2005) Prostate cancer risk and DNA damage: Translational significance of selenium supplementation in a canine model. Carcinogenesis 26(7): 1256-1262. ↵
Together, all of the chemical reactions that take place inside cells, including those that consume or generate energy, are referred to as the cell’s metabolism.
A compound is oxidized when it loses at least one electron.
A metabolic pathway is a series of chemical reactions that takes a starting molecule and modifies it, step-by-step, through a series of metabolic intermediates, eventually yielding a final product.
Atherosclerosis is a condition that occurs when too much plaque builds up in your arteries, causing them to narrow.
Tissues are groups of cells that share a common structure and function and work together.

