Appendix D: Alternative Sweeteners
Sugar substitutes and alternative sweeteners were discussed in chapter 5. This appendix gives additional chemical and health-related information about a variety of sweeteners.
Sugar Alcohols (Polyols, Sugar Replacers)
Sugar(s) can provide a lot of calories and contribute to tooth decay. Thus there are many other compounds that are used as alternatives to sugar that have been developed or discovered. We will first consider sugar alcohols and then the alternative sweeteners in subsequent sections.
Below you can see the molecular structure of three common sugar alcohols: xylitol, sorbitol, and mannitol.
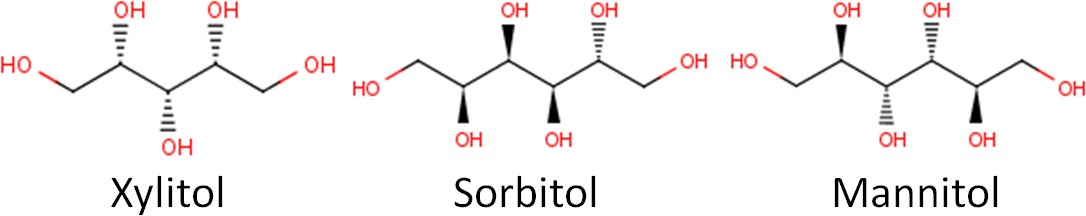
Sugar alcohols are also known as “sugar replacers”, because some in the public might get confused by the name sugar alcohol. Some might think a sugar alcohol is a sweet alcoholic beverage. Another name for them is nutritive sweeteners, which indicates that they do provide calories. Sugar alcohols are nearly as sweet as sucrose but only provide approximately half the calories as shown below. The name polyols also seems to be increasingly used to describe these compounds.
Table D.1 Relative sweetness of monosaccharides, disaccharides, and sugar alcohols[1][2]
|
Sweetener |
Relative Sweetness |
Energy (kcal/g) |
|
Lactose |
0.2 |
4* |
|
Maltose |
0.4 |
4 |
|
Glucose |
0.7 |
4 |
|
Sucrose |
1.0 |
4 |
|
Fructose |
1.2-1.8 |
4 |
|
Erythritol |
0.7 |
0.4 |
|
Isomalt |
0.5 |
2.0 |
|
Lactitol |
0.4 |
2.0 |
|
*Differs based on a person’s lactase activity |
||
Sugars are fermented by bacteria on the surfaces of teeth. This results in a decreased pH (higher acidity) that leads to tooth decay and, potentially, cavity formation (a process officially known as dental caries). The major advantage of sugar alcohols over sugars is that sugar alcohols are not fermented by bacteria on the tooth surface. There is a nice picture of this process in the link below as well as a video explaining the process of tooth decay.
Web Link: Sugar and Dental Caries
Video Link: Tooth Decay (1:06)
Alternative Sweeteners
Alternative sweeteners are simply alternatives to sucrose and other mono- and disaccharides that provide sweetness. Many have been developed to provide zero-calorie or low calorie sweetening for foods and drinks.
Because many of these provide little to no calories, these sweeteners are also referred to as non-nutritive sweeteners (FDA is using high-intensity sweeteners to describe these products[3]). Aspartame does provide calories, but because it is far sweeter than sugar, the small amount used does not contribute meaningful calories to a person’s diet. Until the FDA allowed the use of the term stevia, this collection of sweeteners was commonly referred to as artificial sweeteners, because they were synthetically or artificially produced. However, with stevia, the descriptor artificial can no longer be used to describe these sweeteners. More recently, Luo Han Guo (monk fruit) extracts have also been allowed to be used as another high-intensity sweetener that is not synthesized or artificially produced. The table in the link below summarizes the characteristics of the FDA approved high-intensity sweeteners.
Web Link: FDA High-Intensity Sweeteners
Saccharin
Saccharin is the oldest of the artificial sweeteners. Saccharin was linked to bladder cancer in rats in the late 70’s, but subsequent research did not establish the link in humans. While saccharin might not present as a significant health hazard, you do not want to use it in cooking or baking because it develops a bitter taste.[4]
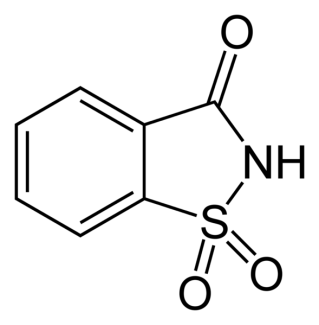
Cyclamate
Cyclamate (sodium cyclamate) is a artificial sweetener that was discovered in 1937. It was banned by the FDA in 1969, primarily due to its questionable safety. Cyclamate is about 30 times sweeter than sucrose, and is often used in combination with other artificial sweeteners. Cyclamate is approved for use in over 80 countries, including those in the European Union and Canada.
Aspartame
Aspartame is made up of 2 amino acids (phenylalanine and aspartate) and a methyl (CH3) group. Aspartame is marketed under the product name NutraSweet ®. The compound is broken down during digestion into the individual amino acids. This is why it provides 4 kcal/g, just like protein.[5]
Because it can be broken down to phenylalanine, products that contain aspartame contain the following message: “Phenylketonurics: Contains phenylalanine.” When heated, aspartame breaks down and loses its sweet flavor.[6]
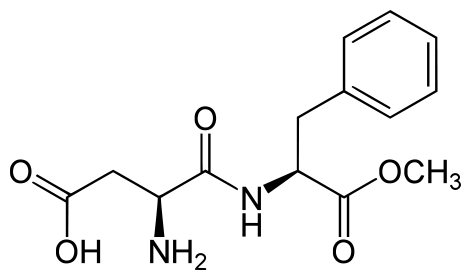
Neotame
Neotame is like aspartame version 2.0. Neotame is structurally identical to aspartame except that it contains an additional side group (bottom of figure below, which is flipped backwards to make it easier to compare their structures). While this looks like a minor difference, it has profound effects on the properties of neotame. Neotame is much sweeter than aspartame and is heat-stable. It can still be broken down to phenylalanine, but such small amounts are used that it is not a concern for those with PKU.[7][8]
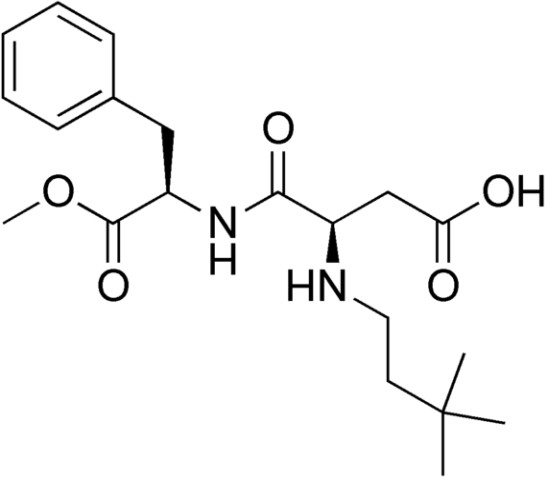
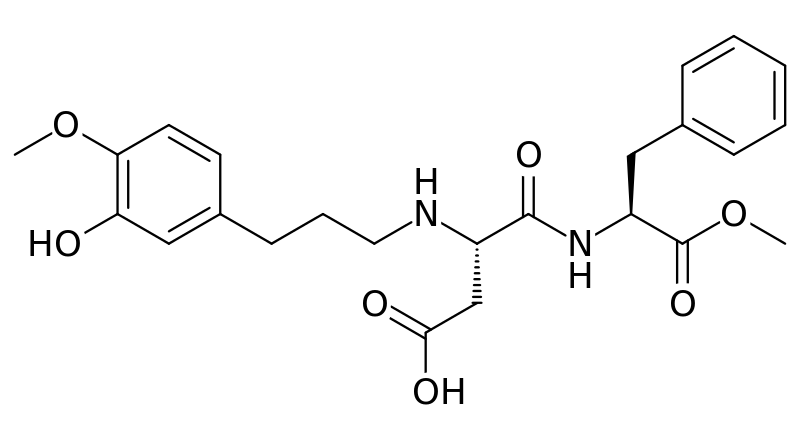
Advantame
The newest, sweetest alternative sweetener approved by the FDA in 2014 is advantame. It is heat-stable and does not have a trade name yet.[9] Notice it also has a similar structure to aspartame and neotame. Like Neotame, it can be broken down to phenylalanine, but such small amounts are used that it is not a concern for those with PKU. However, it has a much higher acceptable daily intake than Neotame[10], meaning there is less concern about adverse effects from consuming too much.
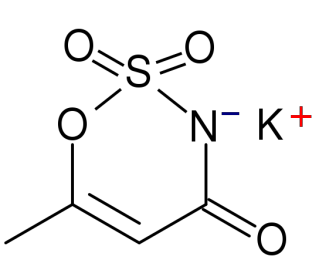
Acesulfame-Potassium (K)
Acesulfame-potassium (K) is not digested or absorbed, therefore it provides no energy or potassium to the body.[11] It is a heat-stable alternative sweetener.
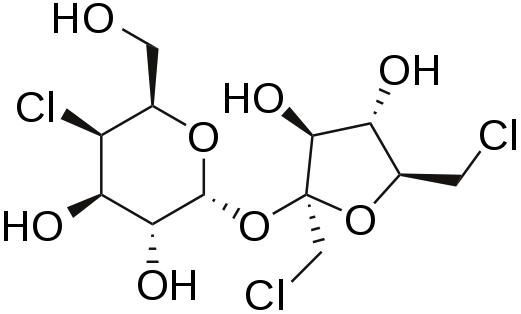
Sucralose
Sucralose is structurally identical to sucrose except that three of the alcohol groups (OH) are replaced by chlorine molecules (Cl). This small change causes sucralose to not be digested and as such is excreted in feces.[12][13] It is a heat-stable alternative sweetener.
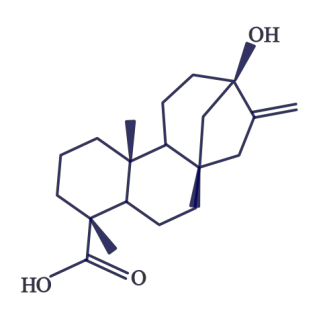
Stevia
Stevia is a heat-stable alternative sweetener derived from a South American shrub, with the leaves being the sweet part. The components responsible for this sweet taste are a group of compounds known as steviol glycosides. The structure of steviol is shown in Figure D.8.
The term glycoside means that there are sugar molecules bonded to steviol. The two predominant steviol glycosides are stevioside and rebaudioside A. Stevia sweeteners have been marketed as natural alternative sweeteners, something that has been stopped by lawsuits as described in the following link.
Web Link: What is natural and who decides?
Luo Han Guo (Monkfruit) Extracts
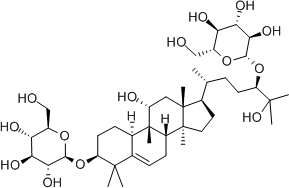
Luo Han Guo (aka Siraitia grosvenrii Swingle, monk fruit) extracts are a newer, natural heat-stable alternative sweetener option derived from a native Chinese fruit. These extracts are sweet because of the mogrosides that they contain.[14] The structure of a mogroside is shown below.
- Wardlaw GM, Hampl J. (2006) Perspectives in Nutrition. New York, NY: McGraw-Hill. ↵
- Whitney E, Rolfes SR. (2008) Understanding Nutrition. Belmont, CA: Thomson Wadsworth. ↵
- http://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm ↵
- Byrd-Bredbenner C, Moe G, Beshgetoor D, Berning J. (2009) Wardlaw's Perspectives in Nutrition. New York, NY: McGraw-Hill. ↵
- Byrd-Bredbenner. et al. (2009) ↵
- Whitney, Rolfes. (2008) ↵
- Whitney, Rolfes. (2008) ↵
- Byrd-Bredbenner, et al. (2009) ↵
- http://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm ↵
- Byrd-Bredbenner, et al. (2009) ↵
- Whitney, Rolfes. (2008) ↵
- Whitney, Rolfes. (2008) ↵
- Byrd-Bredbenner, et al. (2009) ↵
- http://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm397725.htm ↵
A Calorie is a unit of food energy. 1 kilocalorie = 1 Calorie = 1000 calories
Sugar alcohols (such as sorbitol, xylitol, and glycerol) are industrially synthesized derivatives of monosaccharides. Sugar alcohols are often used in place of table sugar to sweeten foods as they are incompletely digested and absorbed, and therefore less caloric.
The Federal Food, Drug, and Cosmetic Act of 1938 gives the FDA authority over food ingredients. The FDA enforces the safety of domestic and imported foods. It also monitors supplements, food labels, claims that corporations make about the benefits of products, and pharmaceutical drugs. Sometimes, the FDA must recall contaminated foods and remove them from the market to protect public health.
Artificial sweeteners are zero calorie or low-calorie replacements for sugar that have been manufactured. They are not nutrients and have no nutritious value.
Amino acids are the building blocks of proteins, simple subunits composed of carbon, oxygen, hydrogen, and nitrogen.

