Chapter 6: Protein
6.4 Protein’s Functions in the Body
University of Hawai‘i at Mānoa Food Science and Human Nutrition Program
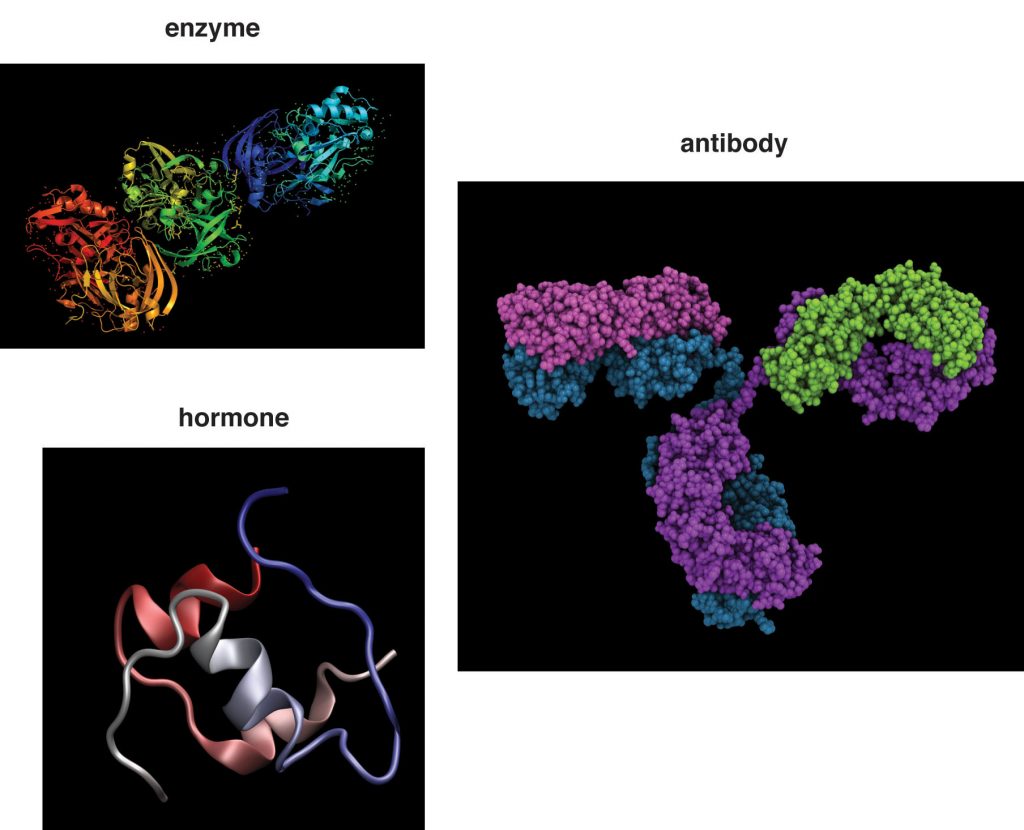
Structure and Motion
More than one hundred different structural proteins have been discovered in the human body, but the most abundant by far is collagen, which makes up about 6 percent of total body weight. Collagen makes up 30 percent of bone tissue and comprises large amounts of tendons, ligaments, cartilage, skin, and muscle. Collagen is a strong, fibrous protein made up of mostly glycine and proline. Within its quaternary structure three peptide strands twist around each other like a rope and then these collagen ropes overlap with others. This highly ordered structure is even stronger than steel fibers of the same size. Collagen makes bones strong, but flexible. Collagen fibers in the skin’s dermis provide it with structure, and the accompanying elastin protein fibrils make it flexible. Pinch the skin on your hand and then let go; the collagen and elastin proteins in skin allow it to go back to its original shape. Smooth-muscle cells that secrete collagen and elastin proteins surround blood vessels, providing the vessels with structure and the ability to stretch back after blood is pumped through them. Another strong, fibrous protein is keratin, which is what skin, hair, and nails are made of. The closely packed collagen fibrils in tendons and ligaments allow for synchronous mechanical movements of bones and muscle and the ability of these tissues to spring back after a movement is complete.
Enzymes
Although proteins are found in the greatest amounts in connective tissues such as bone, their most extraordinary function is as enzymes. Enzymes are proteins that conduct specific chemical reactions. An enzyme’s job is to provide a site for a chemical reaction and to lower the amount of energy and time it takes for that chemical reaction to happen (this is known as “catalysis”). On average, more than one hundred chemical reactions occur in cells every single second and most of them require enzymes. The liver alone contains over one thousand enzyme systems. Enzymes are specific and will use only particular substrates that fit into their active site, similar to the way a lock can be opened only with a specific key. Nearly every chemical reaction requires a specific enzyme. Fortunately, an enzyme can fulfill its role as a catalyst over and over again, although eventually it is destroyed and rebuilt. All bodily functions, including the breakdown of nutrients in the stomach and small intestine, the transformation of nutrients into molecules a cell can use, and building all macromolecules, including protein itself, involve enzymes (see Figure 6.43 “Enzymes Role in Carbohydrate Digestion”).
Video Link: Enzymes (0:49)
In chapter 3, we talked about digestive enzymes, enzymes that catalyze reactions that break down large molecules from food to smaller molecules that can be absorbed into the body.
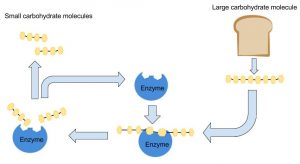
Enzymes’ names commonly end in -ase, and many are named for their substrate. For example, the enzyme amylase cleaves bonds found in amylose and amylopectin.
Hormones
Many hormones are proteins. A hormone is a compound that is produced in one tissue, released into circulation, then has an effect on a different organ. Organs in the body that produce hormones are known as endocrine glands. When an endocrine gland is stimulated, it releases a hormone. The hormone is then transported in the blood to its target cell, where it communicates a message to initiate a specific reaction or cellular process. For instance, after you eat a meal, your blood glucose levels rise. In response to the increased blood glucose, the pancreas releases the hormone insulin. Insulin tells the cells of the body that glucose is available and to take it up from the blood and store it or use it for making energy or building macromolecules. A major function of hormones is to turn enzymes on and off, so some proteins can even regulate the actions of other proteins. While not all hormones are made from proteins, many of them are.
Video Link: Hormones (1:02)
Fluid Balance
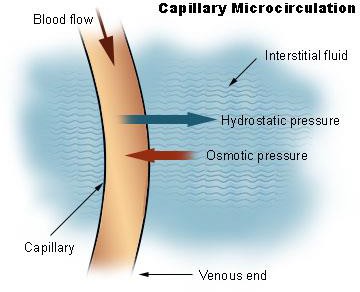
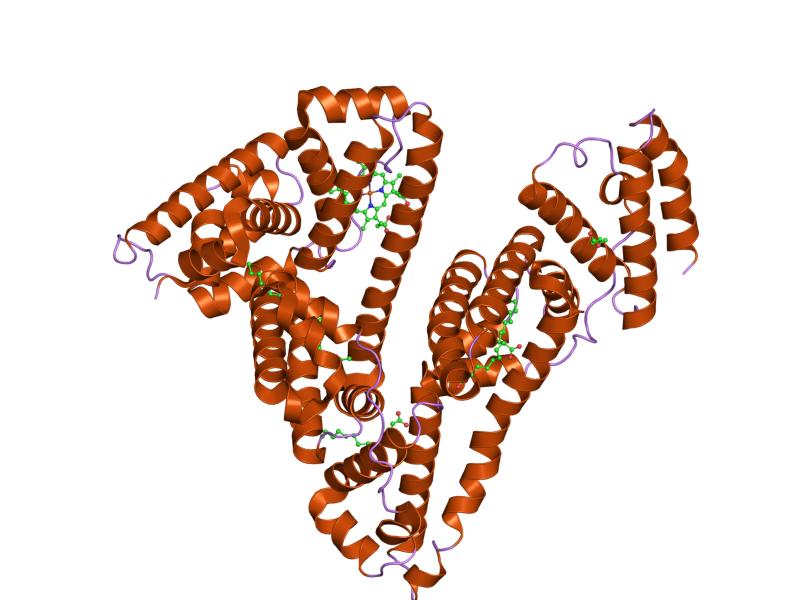
Proper protein intake enables the basic biological processes of the body to maintain the status quo in a changing environment. Fluid balance refers to maintaining the distribution of water in the body. If too much water in the blood suddenly moves into a tissue, the results are swelling and, potentially, cell death. Water always flows from an area of high concentration to one of a low concentration. As a result, water moves toward areas that have higher concentrations of other solutes, such as proteins and glucose. To keep the water evenly distributed between blood and cells, proteins continuously circulate at high concentrations in the blood. The most abundant protein in blood is the butterfly-shaped protein known as albumin (Figure 6.45). Albumin’s presence in the blood makes the protein concentration in the blood similar to that in cells. Therefore, fluid exchange between the blood and cells is not in the extreme, but rather is minimized to preserve the status quo.
Acid-Base Balance
Protein is also essential in maintaining proper pH balance (the measure of how acidic or basic a substance is) in the blood. Blood pH is maintained between 7.35 and 7.45, which is slightly basic. Even a slight change in blood pH can affect body functions. Recall that acidic conditions can cause protein denaturation, which stops proteins from functioning. The body has several systems that hold the blood pH within the normal range to prevent this from happening. One of these is the circulating albumin. Albumin is slightly acidic, and because it is negatively charged it balances the many positively charged molecules, such as protons (H+), calcium, potassium, and magnesium which are also circulating in the blood. Albumin acts as a buffer against abrupt changes in the concentrations of these molecules, thereby balancing blood pH and maintaining the status quo. The protein hemoglobin also participates in acid-base balance by binding and releasing protons.
Transport
Albumin and hemoglobin also play a role in molecular transport. Albumin chemically binds to hormones, fatty acids, some vitamins, essential minerals, and drugs, and transports them throughout the circulatory system. Each red blood cell contains millions of hemoglobin molecules that bind oxygen in the lungs and transport it to all the tissues in the body. A cell’s plasma membrane is usually not permeable to large polar molecules, so to get the required nutrients and molecules into the cell many transport proteins exist in the cell membrane. Some of these proteins are channels that allow particular molecules to move in and out of cells. Others act as one-way taxis and require energy to function.
Protection
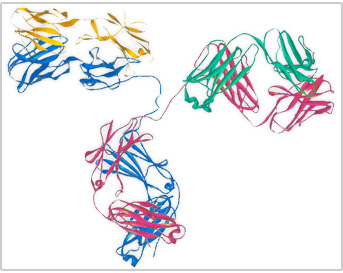

An antibody is a protein made by the immune system to protect against infection. Antibodies are made up of two longer (heavy) amino acid chains and two shorter (light) amino acid chains. These amino acid chains come together to form two antigen-binding sites. An antigen is a substance recognized by antibodies, and may be a bacteria, virus, fungus, parasite, or even some chemicals. When an antibody binds antigen, the immune system is activated to eliminate the infection. Sometimes antibodies are mistakenly produced against harmless substances, such as in allergies and in autoimmune disorders.
Earlier we discussed that the strong collagen fibers in skin provide it with structure and support. The skin’s dense network of collagen fibers also serves as a barricade against harmful substances. The immune system’s attack and destroy functions are dependent on enzymes and antibodies, which are also proteins. An enzyme called lysozyme is secreted in the saliva and attacks the walls of bacteria, causing them to rupture. Certain proteins circulating in the blood can be directed to build a molecular knife that stabs the cellular membranes of foreign invaders. The antibodies secreted by the white blood cells survey the entire circulatory system looking for harmful bacteria and viruses to surround and destroy. Antibodies also trigger other factors in the immune system to seek and destroy unwanted intruders.
Wound Healing and Tissue Regeneration
Proteins are involved in all aspects of wound healing, a process that takes place in three phases: inflammatory, proliferative, and remodeling. For example, if you were sewing and pricked your finger with a needle, your flesh would turn red and become inflamed. Within a few seconds bleeding would stop. The healing process begins with proteins such as bradykinin, which dilate blood vessels at the site of injury. An additional protein called fibrin helps to secure platelets that form a clot to stop the bleeding. Next, in the proliferative phase, cells move in and mend the injured tissue by installing newly made collagen fibers. The collagen fibers help pull the wound edges together. In the remodeling phase, more collagen is deposited, forming a scar. Scar tissue is only about 80 percent as functional as normal uninjured tissue. If a diet is insufficient in protein, the process of wound healing is markedly slowed.
While wound healing takes place only after an injury is sustained, a different process called tissue regeneration is ongoing in the body. The main difference between wound healing and tissue regeneration is in the process of regenerating an exact structural and functional copy of the lost tissue. Thus, old, dying tissue is not replaced with scar tissue but with brand new, fully functional tissue. Some cells (such as skin, hair, nails, and intestinal cells) have a very high rate of regeneration, while others, (such as heart-muscle cells and nerve cells) do not regenerate at any appreciable levels. Tissue regeneration is the creation of new cells (cell division), which requires many different proteins including enzymes that synthesize RNA and proteins, transport proteins, hormones, and collagen. In a hair follicle, cells divide and a hair grows in length. Hair growth averages 1 centimeter per month and fingernails about 1 centimeter every one hundred days. The cells lining the intestine regenerate every three to five days. Protein-inadequate diets impair tissue regeneration, causing many health problems including impairment of nutrient digestion and absorption and, most visibly, hair and nail growth.
Energy Production
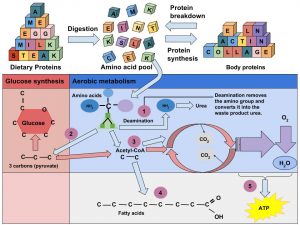
Some of the amino acids in proteins can be disassembled and used to make energy (Figure 6.48 “Amino Acids Used for Energy”). Only about 10 percent of dietary proteins are catabolized each day to make cellular energy. The liver is able to break down amino acids to the carbon skeleton, which can then be fed into the citric acid cycle. This is similar to the way that glucose is used to make ATP. If a person’s diet does not contain enough carbohydrates and fats their body will use more amino acids to make energy, which compromises the synthesis of new proteins and destroys muscle proteins. Alternatively, if a person’s diet contains more protein than the body needs, the extra amino acids will be broken down and transformed into fat.
The fourth (quaternary) level of protein structure is achieved when two or more amino acid chains combine to make one larger functional protein.
The chemical reactants to which an enzyme binds are called the enzyme’s substrates.
The location within the enzyme where the substrate binds is called the enzyme’s active site.
Nutrients are substances the body needs to stay healthy.
Solutes are substances dissolved in a fluid (the solvent).
The most abundant protein in blood is the butterfly-shaped protein known as albumin. Albumin’s presence in the blood makes the protein concentration in the blood similar to that in cells.
Denaturation refers to the physical changes that take place in a protein exposed to abnormal conditions in the environment. Heat, acid, high salt concentrations, alcohol, and mechanical agitation can cause proteins to denature. When a protein denatures, its complicated folded structure unravels, and it becomes just a long strand of amino acids again.
Acid–base balance refers to the balance between input (intake and production) and output (elimination) of hydrogen ion.
Source - https://www.sciencedirect.com/topics/medicine-and-dentistry/acid-base-balance
Vitamins are organic compounds that must be taken in from the diet.
Minerals are solid inorganic substances that form crystals and are classified depending on how much of them we need. Trace minerals such as zinc, iron, or iodine are only required in a few milligrams or less per day. Major minerals such as calcium, sodium, and potassium are required in hundreds of milligrams per day.
An antibody is a protein produced by the body's immune system when it detects harmful substances, called antigens. Examples of antigens include microorganisms (bacteria, fungi, parasites, and viruses) and chemicals.
Source - https://medlineplus.gov/ency/article/002223.htm
An antigen is a substance that activates the immune response, causing production of antibodies.

